by an experienced Chemistry Tutor and Examiner
Posted October 2024
This provides some top tips on how best to revise for Chemistry at GCSE/IGCSE and A Level.
When it comes to revising for your GCSE/IGCSE in Chemistry it is important to remember that a technique that works for one person or subject may not work for all. It is a trial and error process to determine the best techniques for you. Below are a few tips that you can try as a way to aid you when it comes to the question of ‘how to revise for Chemistry’.
Tip #1 – Start early and know the basics
Most students leave their revision till either just before or after their mock exams, some even leave it till Easter holidays. The earlier you start, the less cramming you will need to do. Start at the beginning of Year 11, go right back at the Unit 1 even if you think you know the contents. The more solid your foundational knowledge is, the easier you will find understanding the more complex/abstract concepts in chemistry. Everything in chemistry builds upon itself, so make sure you know the basics well.
Tip #2 – Make a Chemistry Dictionary
Science has a language of its own, especially Chemistry. Think about building a dictionary of key terms you come across throughout your GCSE years. Write them down and their meanings. This is something that you can build upon and refer to, not only throughout your GCSE/IGCSE but also A-level years and beyond. You will find this to be a massive help in those moments where your brain just has a forgetful moment, or you are in a rush to complete an answer. When I started my degree in chemistry, this was the first bit of advice the Head of Department at the university I attended gave all students, and it was a lifesaver at times.
Tip #3 – Periodic Table
The periodic table will play a major portion of your GCSE/IGCSE exam, so becoming familiar with it and where elements are located can save you time on your exam. Now I am not saying learn it off by heart, but having a good idea of where certain elements can be found, will save you from searching for them during an exam. If you spend 30 seconds looking for Iron (Fe) on the periodic table during your exam, that is 30 seconds you could have spent writing or doing a calculation later on. Knowing that Iron is a transition metal, and its rough location is in the centre of the periodic table will make a massive difference.
Additionally, there are some elements that are used continually throughout GCSE/IGCSE like Carbon (C), Oxygen (O), Nitrogen (N), Chlorine (Cl) just to name a few. So, knowing their atomic number or mass number can also save you precious time.
Tip #4 – 6 mark questions – Answer the question
Practice, practice, practice these. It is so much easier to get 3/6 or 4/6 then it can be to get 1/2 or 2/3 at times. Learn how to decipher what is being asked of you. Each exam board has their own way of asking questions, so know what each question phrase means for your exam board, so you know how to answer the question.
Then ANSWER THE QUESTION. That is the most important thing. So many students waffle away writing big answers but never hit any of the indicative points, and score very low. Knowing what your exam board is asking of you will go a long way.
Learning to answer 6 mark questions will also help instil those concepts in your memory, which you can later use to answer short or multiple choice questions. There will always be an application for that knowledge in your exams, so practicing 6 mark questions will not only help you develop a plan of action but develop your understanding of those concepts.
Also, use the mark schemes to help. If you are unsure on how to read a mark scheme for the 6 mark questions, ask your teacher or tutor. They will be able to explain to you on how to read them and learn how to gain full marks on an exam.
Tip #5 – Bonding
Bonding can be a very abstract concept for some people. Knowing the difference between ionic and covalent bonding or metallic and ionic substance for conduction of electricity, are very commonplace where students lose their marks.
Ionic bonding is the transfer of electrons which forms ions. Covalent bonding is the sharing of electrons.
Ionic substances can conduct electricity when molten or in solution due to the free moving IONS, whereas metallic substance have delocalised ELECTRONS, which carry the electrical current.
Tip #6 – Don’t overthink
It can be very easy to overthink an answer. Sometimes the simplest of answers is the right answer.
By now you will have found techniques that work best for you when it comes to revision, but here are some tips to aid your revision.
Tip #1 – Remember your GCSE content
Some students are often told by a teacher to forget everything they learnt in their GCSE Chemistry because it was wrong and A-Level Chemistry is real Chemistry. Don’t do this. The information you learnt throughout your GCSEs is fundamental in understanding chemistry. The thing that may have changed is how you are expected to present that information, ie orbitals not shells.
You will find however, that some questions do ask you to draw upon your GCSE knowledge, for example dot and cross diagrams or fractional distillation. So it is important that you still have that knowledge.
Tip #2 – Make a Chemistry Dictionary
Science has a language of its own, especially Chemistry. When I started my degree in chemistry, this was the first bit of advice the Head of Department at the university I attended gave all students, and it was a lifesaver at times, was to make a dictionary of new chemistry terms as they come up.
Tip #3 – Calculations
Practice all your calculations, but if you get stuck and are able convert the data you have to MOLES. Most likely if you can’t go any further, there will be marks awarded for converting to moles. So attempting to gain even those marks when unable to do the full calculation could be the difference in a grade boundary.
Some calculations you will have on exams could have multiple routes to achieve the desired answer. It is left up to the student to figure this out.
Tip #4 – 6 mark questions – Answer the question
Read the question and answer it!
As an examiner the number of times I have come across answers that are well scripted but do not answer what is being asked is endless. When a question lists out items it wants you to write about, make sure you include that information. Most exam boards will expect 2-3 points for each point being asked. So, if you are asked about safety precautions, give 3-4 with reasoning. More information is required for A-Level answers, it is not always 1 mark 1 answer.
Tip #5 – Diagrams
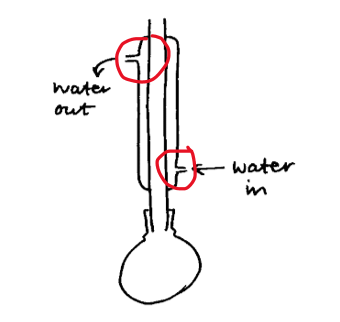
If you are asked to draw a cross-section of apparatus and label, make sure you do both. A cross-section is how you would see the apparatus from the side, so do not close off openings, you will not gain the marks. For example, when drawing a cross-section of reflux or distillation, the water in and out on the condenser need to be open and correctly labelled. So many students lose marks for not labelling when asked to. Simple marks lost.
Example of diagram that is RIGHT
Example of diagram that is WRONG
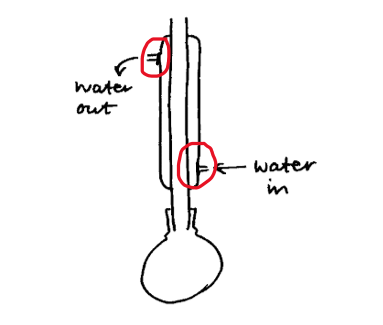
Tip #6 – Don’t overthink
It can be very easy to overthink an answer. Sometimes the simplest of answers is the right answer.
But also, do not LIST. A right answer can immediately be cancelled out by a wrong answer from a list. While getting your knowledge on paper is important, how you do it is extremely important.
If you write an answer, you think is wrong and cross it out then write another answer, your crossed out answer cannot be looked at. So, when crossing out an answer make sure you want to do that.
Tip #7 – Organic Mechanisms
Regardless of what exam board you write, there will be an organic mechanism of some kind. Be careful with your arrows. They must:
We hope that you have enjoyed reading our blog on how best to revise for Chemistry at GCSE, IGCSE and A Level. For top tips on other subjects that you might also be studying, please visit our main blog page, via the button below. If you would be interested in attending a revision course for Chemistry, please contact us today for further information or apply via our online application forms.
Subscribe today for the latest news and updates
Oxford Science Studies is a trading name of Fieldwork Education Ltd, Registered Company Number 03299897.
Copyright @ 2024 Nord Anglia Education
Nord Anglia Education
4th Floor, Nova South
160 Victoria Street
London, United Kingdom
SW1E 5LB
+44(0)207 131 0000
enquiries@nordanglia.com